
Castle Creek Biosciences’ proprietary autologous fibroblast technology is the foundation of our approach for developing personalized, targeted and re-dosable cell-based, gene therapy candidates for monogenic chronic diseases, with an initial focus on skin and connective tissue disorders.
Fibroblasts synthesize extracellular matrix proteins like collagen, which provide structure and support to the skin. Fibroblasts have unique qualifications for localized delivery to gene targets in skin and connective tissues.
This technology was used to develop D-Fi, also known as FCX-007, (dabocemagene autoficel), an autologous gene therapy candidate for the treatment of dystrophic epidermolysis bullosa (DEB)—a devastating progressive, painful and debilitating rare genetic skin disorder. DEB is caused by a mutation in the COL7A1 gene, leading to a deficiency of normal type VII collagen (COL7) protein, impairing the connection between the epidermis and the dermis.
D-Fi is comprised of a patient’s own dermal fibroblasts, which are genetically modified with a self-inactivating (SIN) lentiviral vector (LV) containing the COL7A1 gene to express COL7. D-Fi is locally administered by intradermal injection into chronic wounds where the COL7 protein can support the formation of anchoring fibrils in the skin.
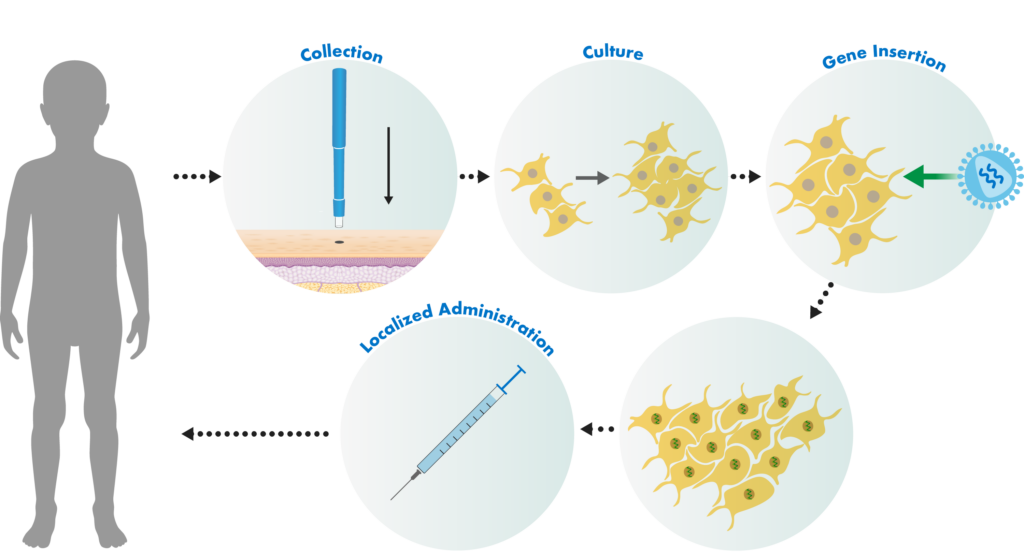
Castle Creek Biosciences’ autologous fibroblast technology uses our patented manufacturing process. Small skin biopsies are taken from patients. Those cells are stored and used to develop doses of cell-based gene therapies that are tailored to each patient.
The fibroblast cells in the biopsies are separated from other cell types and then grown in a special fluid in a lab. Those cells are then exposed to a lentiviral vector. The lentiviral vector is genetically engineered to insert a correct copy of the type COL7 gene into the patient’s cells.
The cells are then locally administered to active wounds, with the goal of restoring skin integrity. As part of this process, a personalized cell bank of genetically modified autologous fibroblasts is cryogenically stored from a single biopsy, serving as a repository for a patient’s long-term therapeutic needs.
1 M. P. Marinkovich, MD, N. Ehsani-Chimeh, MD, N. Nguyen, S. Moncrief, PhD, V. K. Dailey, M. Chakiath, A. Elayadi, PhD, S. Krishnan, MS, and J. Maslowski, MS. Pre-Clinical Development of a Genetically-Modified Human Dermal Fibroblast (FCX-007) for the Treatment of Recessive Dystrophic Epidermolysis Bullosa. Presented at the American Society of Human Genetics Annual Meeting, Baltimore, Maryland, October 8, 2015.
2 V.K. Dailey, M. Chakiath, A. Elayadi, PhD, S. Krishnan, MS, J. Maslowski, MS, M. P. Marinkovich, MD. Development of a Genetically-Modified Human Dermal Fibroblast for the Treatment of Recessive Dystrophic Epidermolysis Bullosa. Presented at the European Society of Human Genetics, Glasgow, Scotland, United Kingdom, June 8, 2015.
Castle Creek Biosciences’ product candidates are investigational. None of our investigational gene therapies have received marketing approval by the U.S. Food and Drug Administration or any other regulatory agency.